| Coordination Complexes | Werner's Thoery of Coordination Complexes | Typical Ligands |
| Typical Coordination Numbers | Lewis Acid-Lewis base Approach to Bonding in Complexes | |
Coordination compounds, such as the FeCl4- ion and CrCl3 6 NH3, are called such because they contain ions or molecules linked, or coordinated, to a transition metal. They are also known as complex ions or coordination complexes because they are Lewis acid-base complexes. The ions or molecules that bind to transition-metal ions to form these complexes are called ligands (from Latin, 'to tie or bind'). The number of ligands bound to the transition metal ion is called the coordination number.
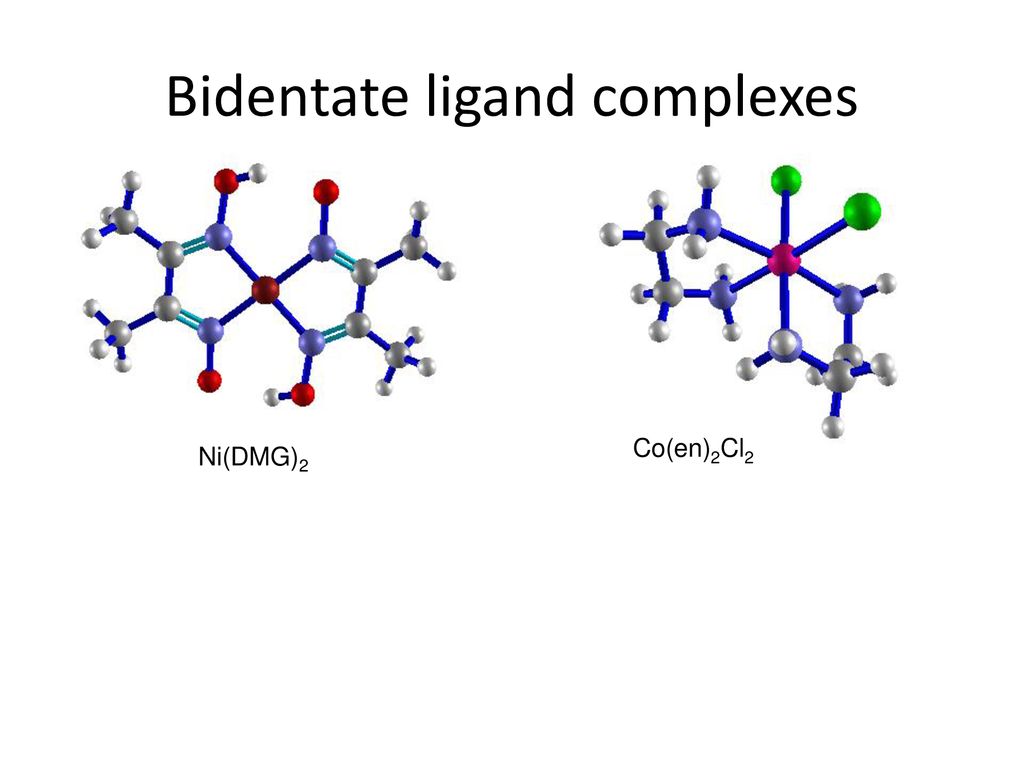
Dimethylglyoxime (dmg) is a bidentate ligand chelating large amounts of metals. Only two dmg molecules per metal center are needed, because Ni (dmg)22 + has a square-planar geometry. To the aqueous array, add 1 per cent dmg. What is the role of DMG in analytical chemistry? DMG N,N-DIMETHYLGLYCINE Find entries where: DMG is present as a standalone ligand. DMG as a non-polymer is covalently linked to polymer or other heterogen groups. Ni (DMG)2 (s) is a red precipitate As you can see nickel has not been oxidized, nickel is not oxidized as the Ni2+ ion only forms a complex. DMG has to be in an alcohol solution because It is the. The other Co (III) atom is coordinated by four nitrogen atoms of two cis nonplanar ligands, dmg2−, dmg H −, and two oxygen atoms of the other dmg2− and dmg H − ligands. There are three N‐O bridged ligands namely two dmg2− and one dmg H − between two Co (III) atoms.
Although coordination complexes are particularly important in the chemistry of the transition metals, some main group elements also form complexes. Aluminum, tin, and lead, for example, form complexes such as the AlF63-, SnCl42- and PbI42- ions.
Alfred Werner developed a model of coordination complexs which explains the following observations.
- At least three different cobalt(III) complexes can be isolated when CoCl2 is dissolved in aqueous ammonia and then oxidized by air to the +3 oxidation state. A fourth complex can be made by slightly different techniques. These complexes have different colors and different empirical formulas.
| CoCl3 6 NH3 | orange-yellow |
| CoCl3 5 NH3 H2O | red |
| CoCl3 5 NH3 | purple |
| CoCl3 4 NH3 | green |
- The reactivity of the ammonia in these complexes has been drastically reduced. By itself, ammonia reacts rapidly with hydrochloric acid to form ammonium chloride.
NH3(aq) + HCl(aq) NH4+(aq) + Cl-(aq)
These complexes don't react with hydrochloric acid, even at 100oC.
- Solutions of the Cl- ion react with Ag+ ion to form a white precipitate of AgCl.
Ag+(aq) + Cl-(aq) AgCl(s)
When excess Ag+ ion is added to solutions of the CoCl3 6 NH3 and CoCl3 5 NH3 H2O complexes, three moles of AgCl are formed for each mole of complex in solution, as might be expected. However, only two of the Cl- ions in the CoCl3 5 NH3 complex and only one of the Cl- ions in CoCl3 4 NH3 can be precipitated with Ag+ ions.
- Measurements of the conductivity of aqueous solutions of these complexes suggest that the CoCl3 6 NH3 and CoCl3 5 NH3 H2O complexes dissociate in water to give a total of four ions. CoCl3 5 NH3 dissociates to give three ions, and CoCl3 4 NH3 dissociates to give only two ions.
Werner explained these observations by suggesting that transition-metal ions such as the Co3+ ion have a primary valence and a secondary valence. The primary valence is the number of negative ions needed to satisfy the charge on the metal ion. In each of the cobalt(III) complexes previously described, three Cl- ions are needed to satisfy the primary valence of the Co3+ ion.
The secondary valence is the number of ions of molecules that are coordinated to the metal ion. Werner assumed that the secondary valence of the transition metal in these cobalt(III) complexes is six. The formulas of these compounds can therefore be written as follows.
| [Co(NH3)63+][Cl-]3 | orange-yellow |
| [Co(NH3)5(H2O)3+][Cl-]3 | red |
| [Co(NH3)5Cl2+][Cl-]2 | purple |
| [Co(NH3)4Cl2+][Cl-] | green |
The cobalt ion is coordinated to a total of six ligands in each complex, which satisfies the secondary valence of this ion. Each complex also has a total of three chloride ions that satisfy the primary valence. Some of the Cl- ions are free to dissociate when the complex dissolves in water. Others are bound to the Co3+ ion and neither dissociate nor react with Ag+.
The [Co(NH3)6]Cl3 complex dissociates in water to give a total of four ions, and all three Cl- ions are free to react with Ag+ ion.
One of the chloride ions is bound to the cobalt in the [Co(NH3)5Cl]Cl2 complex. Only three ions are formed when this compound dissolves in water, and only two Cl- ions are free to precipitate with Ag+ ions.
| H2O | |
| [Co(NH3)5Cl][Cl]2(s) | Co(NH3)5Cl2+(aq) + 2 Cl-(aq) |
Once again, the three Cl- ions are free to dissociate when [Co(NH3)5(H2O)]Cl3 dissolves in water, and they precipitate when Ag+ ions are added to the solution.
| H2O | |
| [Co(NH3)5(H2O)]Cl3(s) | Co(NH3)5(H2O)3+(aq) + 3 Cl-(aq) |
Two of the chloride ions are bound to the cobalt in [Co(NH3)4Cl2]Cl. Only two ions are formed when this compound dissolves in water, and only one Cl- ion is free to precipitate with Ag+ ions.
Werner assumed that transition-metal complexes had definite shapes. According to his theory, the ligands in six-coordinate cobalt(III) complexes are oriented toward the corners of an octahedron, as shown in the figure below.
Any ion or molecule with a pair of nonbonding electrons can be a ligand. Many ligands are described as monodentate (literally, 'one-toothed') because they 'bite' the metal in only one place. Typical monodentate ligands are given in the figure below.
Other ligands can attach to the metal more than once. Ethylenediamine (en) is a typical bidentate ligand.
Each end of this molecule contains a pair of nonbonding electrons that can form a covalent bond to a metal ion. Ethylenediamine is also an example of a chelating ligand. The term chelate comes from a Greek stem meaning 'claw.' It is used to describe ligands that can grab the metal in two or more places, the way a claw would.
Linking ethylene- diamine fragments gives tridentate ligands and tetradentate ligands, such as diethylenetriamine (dien) and triethylenetetramine (trien). Adding four -CH2CO2- groups to an ethylenediamine framework gives a hexadentate ligand, which can single-handedly satisfy the secondary valence of a transition-metal ion.
Typical Coordination Numbers
Transition-metal complexes have been characterized with coordination numbers that range from 1 to 12, but the most common coordination numbers are 2, 4, and 6. Examples of complexes with these coordination numbers are given in the table below.
Examples of Common Coordination Numbers
| Metal Ion | Ligand | Complex | Coordination Number | |
| Ag+ | + | 2 NH3 | Ag(NH3)2+ | 2 |
| Ag+ | + | 2 S2O32- | AgCl2- | 2 |
| Ag+ | + | 2 Cl- | Ag(S2O3)23- | 2 |
| Pb2+ | + | 2 OAc- | Pb(OAc)2 | 2 |
| Cu+ | + | 2 NH3 | Cu(NH3)2+ | 2 |
| Cu2+ | + | 4 NH3 | Cu(NH3)42+ | 4 |
| Zn2+ | + | 4 CN- | Zn(CN)42- | 4 |
| Hg2+ | + | 4 I- | HgI42- | 4 |
| Co2+ | + | 4 SCN- | Co(SCN)42- | 4 |
| Fe2+ | + | 6 H2O | Fe(H2O)62+ | 6 |
| Fe3+ | + | 6 H2O | Fe(H2O)63+ | 6 |
| Fe2+ | + | 6 CN- | Fe(CN)64- | 6 |
| Co3+ | + | 6 NH3 | Co(NH3)63+ | 6 |
| Ni2+ | + | 6 NH3 | Ni(NH3)62+ | 6 |
Note that the charge on the complex is always the sum of the charges on the ions or molecules that form the complex.
Cu2+ + 4 NH3 Cu(NH3)42+
Pb2+ + 2 OAc- Pb(OAc)2
Fe2+ + 6 CN- Fe(CN)64-
Dmg Ligand Strength
Note also that the coordination number of a complex often increases as the charge on the metal ion becomes larger.

Cu+ + 2 NH3 Cu(NH3)2+
Cu2+ + 4 NH3 Cu(NH3)42+